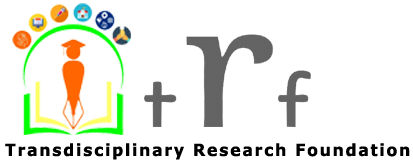Economic Evaluation Studies of Pharmaceutical products and Medical Equipments
Health economic evaluation studies provide extensive information to decision makers as well as innovators/manufacturers of pharmaceuticals & medical devices for efficient use of available resources for maximizing health benefits for the end beneficiary as well as making reasonable profits for the innovator. An ‘economic evaluation study’ is one key part of health economics, and is a tool for comparisons associated with costs and consequences of different health interventions, both at the development as well as at the implementation stage. ‘Health technology assessment’ is one of the key techniques under economic evaluation that is well adapted by developed countries & is being acknowledged & implemented in developing countries. In a few countries, different methods of economic evaluation have been adopted for decision making, concerned with public subsidies for the procurement of medicines & medical devices. India is amidst design & implementation of several macro/micro-level strategies to improve public/private healthcare services for its large population, including investing in public-private-partnership enabled healthcare services. Any prospects for future growth and development in this field will necessitate greater synergies with respect to economic efficiencies in the healthcare system particularly keeping in mind of India’s rapid healthcare inflation; increasing rates of chronic conditions because of rapid urbanisation; an ever increasing aging population; and increasing technology diffusion.
When it comes to institutions (Government or Non-government), “Pharmacoeconomics is increasingly being used by decision makers when selecting an appropriate treatment for patients“, which, are studies conducted to evaluate the cost (expressed in monetary terms) and effects (expressed in terms of monetary value, efficacy or enhanced quality of life) of a pharmaceutical or medical product. The entire process finds consonance with both health policy bearers and leadership teams in pharmaceutical/medical device manufacturing organizations since assessment of effectiveness and safety of new medical interventions (including pharmaceuticals/medical devices) has always remained a hallmark of evidence-based medicine (EBM). More recently, costs associated with these new innovations have become an increasingly important topic for discussion. Pharmacoeconomics, a subset of healthcare economics, “identifies, measures, and compares the costs and consequences of pharmaceutical products and services”. Clinicians and other decision makers can use pharmacoeconomic evaluations to assess and compare the total costs of treatment options with the outcomes associated with these options.
Health economic studies provide information to decision makers as well as manufacturer organisations for efficient use of available resources for maximizing health benefits as well as being economically viable for the innovators. Economic evaluation is merely one part of health economics, and is a tool for comparing costs and consequences of different interventions. Health technology assessment is another technique for economic evaluation that is well adapted by developed countries as well as picking up recently in developing countries. The traditional classification of economic evaluation includes cost-minimization, cost-effectiveness analysis, cost-utility analysis, and cost-benefit analysis. There has been uncertainty in the conduct of such economic evaluations in India, due to some hesitancy with respect to the adoption of their guidelines. The biggest challenge in this evolutionary method is lack of understanding of methods in current use by all those involved in both provision and purchasing of health care.




The Societal Importance of Economic Evaluation Studies
Health care costs around the world have been increasing each year, in certain countries, substantially more than the average rate of inflation and India is no exception. Costs per person in the India are may not be comparable to countries such as the United States or Europe but these ‘out-of-pocket expenses’ spent on healthcare contributes to high incidences of catastrophic expenditures resulting into absolute poverty. With the advent of biologic medications, revolutionary hepatitis treatments, and advanced cancer therapies, some treatments are now not affordable (for the end-beneficiaries or their families). There is a vital need to understand how limited resources can be used most efficiently and effectively. Clinicians want their patients to receive the best care and outcomes available, but at what cost? Is the added cost worth the added clinical benefit? Pharmacoeconomics is now considered as part of the evaluation process of new treatments.
Relationship of Pharmacoeconomics to Other Types of Research
Pharmacoeconomics is by its nature a multidisciplinary endeavor, and there is no standardized training for pharmacoeconomists. This is a relatively new discipline -the term ‘pharmacoeconomics’ first appeared in the literature in the mid-1980s-yet the concepts and methods are borrowed from other, more established disciplines and research areas. Two main disciplines that are important are health care economics and clinical or humanistic outcomes research. Health care economics encompasses a broad range of topics, including the effects of policy changes (e.g., insurance regulations), supply and demand for health care resources, the and the available supply of healthcare providers. Clinical or humanistic outcomes research is defined as the attempt to identify, measure, and evaluate the end results of health care services. In addition to clinical and economic consequences, outcomes such as patients’ health status and satisfaction with their health care may be assessed. ‘Pharmacoeconomics’ is a type of outcomes research, but not all outcomes research is pharmacoeconomic research. If the research involves both economic and clinical outcome evaluations AND the comparison of pharmacy products or services, it can be termed as a pharmaco-economic study.
ABOUT US
Transdisciplinary Research Foundation or TRF is a Section VIII non profit research focused organisation.
ALL CONTACTS
- C-9/9867, Vasant Kunj, New Delhi-110 070
- Office +91 9910487009
- ranju@tresfoundation.org
- Tresfoundation - Copyright 2021
- ranju@tresfoundation.org